AI drug discovery is transforming pharmaceutical research, dramatically speeding up drug development and enhancing innovation. Yet, the real key to harnessing artificial intelligence effectively lies in high-quality, harmonized data. In this article, we’ll explore why optimized data management is essential in empowering AI-driven drug discovery.
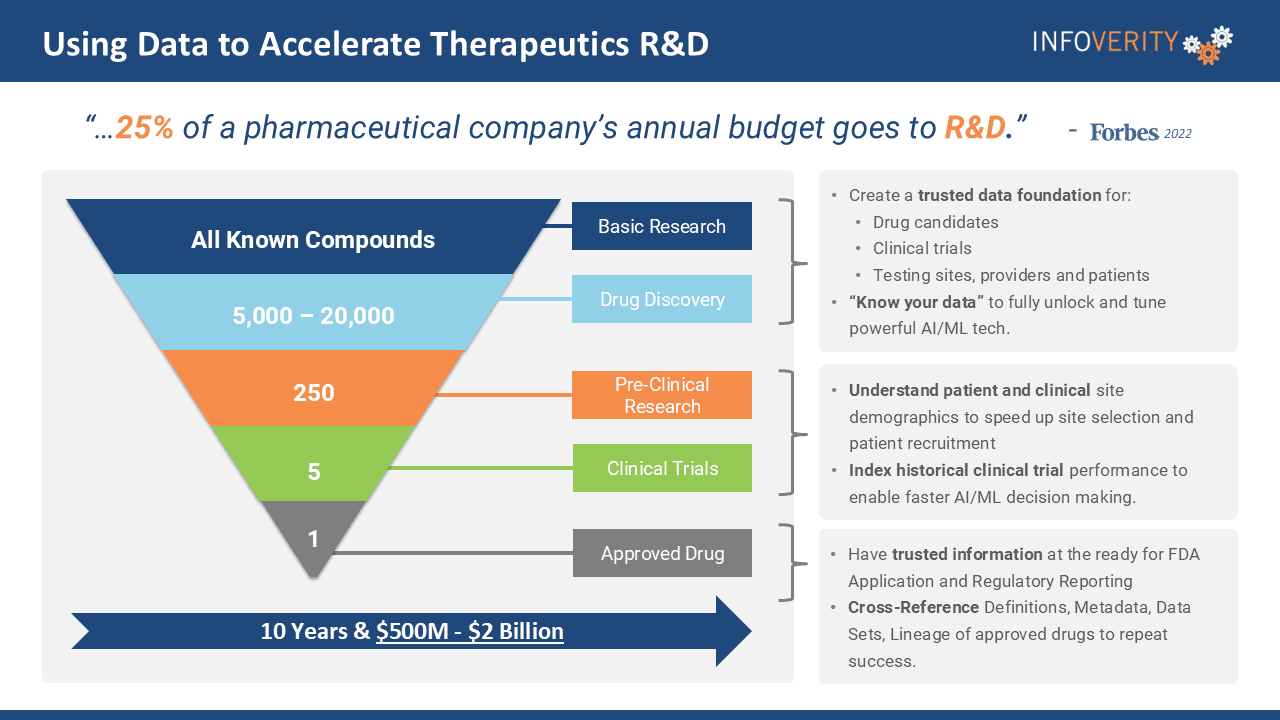
According to Forbes, 25% of a pharmaceutical company’s annual budget is typically spent on research and development (R&D).
It’s a costly endeavor. Drug development timelines can stretch up to 10 years, with as many as 20,000 compounds screened for every one drug that receives approval. This lengthy process often comes with a price tag ranging from $500 million to $2 billion.
Stronger Together: AI Drug Discovery and Quality Data: Table of Contents
Time is money, so there is a growing urgency to expedite the drug development cycle. Even saving a single year in drug development can give companies a critical competitive edge and significantly enhance their return on investment.
Can increasingly ubiquitous AI alleviate the industry’s most pressing challenges? There are early signs that this is the case: industry leaders like Pfizer and Novartis are already getting meaningful results by integrating AI into their research processes.
Let’s examine how the technology is now used in R&D lifecycles.
AI in Drug Discovery: Taking On Many Roles
AI enables a faster time-to-market by streamlining large-scale, exhaustive testing and trial-and-error strategies.
By analyzing massive datasets, the technology can rapidly evaluate and eliminate non-viable compounds early in the discovery pipeline. This reduces time, cost, and the burden of failed testing, saving resources for high-value processes and projects.
Pfizer’s AI Models reportedly achieved a 15% improvement in success rates for compounds entering Phase II, while Novartis’ Target Prioritization reduced early-stage development costs by 40% with AI integration.
Machine learning (ML) and natural language processing (NLP) also help pharma companies gain deeper insights into disease biology and identify ideal patient populations for newly developed treatments. For example, Pfizer partnered with IBM Watson to analyze patient records and match trial eligibility for their “immuno-oncology” research.
Additionally, AI’s predictive modeling capabilities allow researchers to anticipate a compound’s efficacy and safety well before it enters a lab.
Finally, the technology has a broader capacity to uncover hidden patterns, changing the way companies can approach drug discovery.
AlphaFold2 – A Nobel prize Winning AI Model
An excellent example is one half of the 2024 Nobel Prize in Chemistry, awarded to Demis Hassabis and John Jumper for their work on AlphaFold2.
AlphaFold2 is an AI model that solved the 50-year challenge of predicting protein structures from amino acid sequences. The program has helped predict almost all 200 million proteins and has been used by two million people in 190 countries. Among its useful applications, it has informed new research into the enzymatic decomposition of plastic and antibiotic resistance.
Companies also count on AI to handle another hurdle: obtaining regulatory approval. According to McKinsey, Gen AI–enabled intelligence engines are useful on three fronts:
Predicting potential health authority query (HAQ) patterns based on submitted data
Formulating appropriate and timely responses to sponsors
Providing deeper intelligence to submission processes
It’s also a good idea that regulatory bodies like the US FDA and Europe’s EMA both recognize the role of AI in this space, ensuring regulation doesn’t get in the way of innovation.
Last but not least, AI capabilities lend themselves well to post-market surveillance, which is necessary to ensure long-term safety, effectiveness, and practical application. It can mine, flag, and detect patterns of low efficacy and adverse effects.
Interested in transforming your R&D pipeline?
Contact Infoverity today to find out how our end-to-end Master Data Management solutions and other services can turn you into an AI-ready pharmaceutical company.
Clean Data: The Key Driver of AI Drug Discovery
Pharmaceutical firms train and refine their AI models using a variety of data types, including:
- Historical clinical trial results – Previous trial results guide AI in selecting better candidates and improving study design.
- Genomic and molecular data – Genetic and biochemical information support personalized medicine and enable precision targeting of compounds based on biological markers.
- Patient demographics and health outcomes – Population-level attributes and clinical results help identify suitable patient groups and improve trial diversity.
- Trial site performance metrics – Operational data on trial locations informs smarter site selection based on enrollment rates, dropout trends, and data quality.
However, a lack of data hygiene can quickly derail even the most sophisticated models. Poor-quality or inconsistent inputs introduce bias, generate unreliable predictions, and cause key therapeutic opportunities to be missed. To maintain model reliability and regulatory compliance, companies must prioritize:
- Consistency, accuracy, and completeness across all global data sources
- Standardized definitions of key entities, such as patient populations, trial sites, and compounds
- Strong data lineage and audit trails to ensure compliance and transparency
All this to say: AI’s potential is bound by the integrity of the data behind it. No matter what the task is—finding promising compounds, predicting safety risks, or optimizing clinical trials—the quality, breadth, and consistency of the data determine how well this technology can deliver.
Developing AI-Ready Data to Support Pharmaceutical Innovation
To create a data-driven environment where AI thrives, pharmaceutical companies need to adhere to these three pillars of data management as industry best practices:
1. Capture and Integrate
Data should be accessible in one place. In AI drug discovery, this means unifying data from labs, clinical systems, electronic health records (EHRs), and third-party providers.
Data silos are notorious for poor data quality and process inefficiency. They cause further delays as well as errors in analysis and reporting, increasing the cost of drug discovery.
Master Data Management (MDM) technologies prevent this from happening by bringing together disparate R&D platforms, creating a single source of truth for critical domains of data like Product, Solution, Compound, Patients, Trial Sites, etc.. We also provide a change management program to assist companies during this transition and beyond.
2. Curate and Standardize
Data harmonization is necessary to convert fragmented or inaccurate data into useful and accessible information.
Core entities like compounds, patients, and trial sites need to use standardized naming conventions and controlled vocabularies to ensure consistency across systems. Using metadata to tag attributes like Genomic, Clinical, De-Identified, PHI, PII, originating source,and time stamp further improves discoverability and enhances model training quality.
Infoverity brings deep Data Management experience to the table, standardizing critical datasets to ensure consistency, enable observability and monitoring, and support downstream AI applications with cleaner, more reliable data. More than that, we provide metadata management that can reduce storage costs by 30% and time to find data by 50%.
3. Govern and Comply
As regulatory bodies like the FDA and EMA set standards for AI in pharmaceutical workflows, companies must proactively demonstrate data integrity, explainability, and accountability.
Infoverity has its own Data Governance Diagnostic tool and provides governance frameworks that balance innovation with regulatory compliance. Pharmaceutical firms can innovate responsibly, without compromising oversight or quality.
Interested in transforming your R&D pipeline?
Contact Infoverity today to find out how our end-to-end Master Data Management solutions and other services can turn you into an AI-ready pharmaceutical company.



